Heat Exchangers: Chemically Induced Corrosion
These failures result from the complex chemical interaction between the materials of the heat exchanger and the fluids circulated through it. There are seven types of chemically induced corrosion failures: general corrosion, pitting corrosion, stress corrosion, dezincification, galvanic corrosion, crevice corrosion, and condensate grooving.
General Corrosion -- This type of corrosion is characterized by a relatively uniform attack over the tube, tube sheet, or shell, and there may be no evidence that corrosion is taking place. Figure 10 shows a tube that became so thin it actually tore down the middle
Fig. 10. Thinning of tube because of general corrosion.
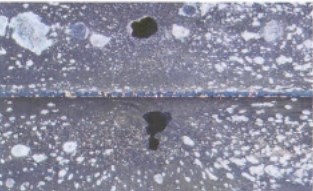
Fairly stable aggressive conditions generate this type of attack. Low pH (less than 7) combined with carbon dioxide or oxygen produces the attack on copper. The blue or bluish-green color on the tubes in Fig. 11 shows the results of carbon dioxide attack on the inside of a copper tube.
Fig, 11 General corrosion inside copper tube because of C0
Various chemicals, such as acids. also produce this type of metal loss. Selecting a material with adequate corrosion resistance for its environment, along with using proper treatment chemicals, maximizes heat exchanger life.
Pitting Corrosion -- Localized pitting is frequently encountered in ferrous and nonferrous metals. It results from the electrochemical potential set up by differences in oxygen concentration within and outside the pit, and is frequently referred to as a concentration cell. The oxygen-starved pit acts as an anode and the unattacked metal surface as a cathode. A small number of pits may be present; however, any one can cause a heat exchanger failure. Pitting corrosion is most likely to occur during shutdown periods a when there is no flow and the environment is most suitable for the buildup of concentration cells. The susceptibility to pitting corrosion is further enhanced by scratches, dirt or scale deposits, surface defects, breaks in protective scale layers. breaks in metal surface films, and grain boundarv conditions. Figure 12 shows an
oxygen pining attack on a copper tube,
. Fig. 12. Oxygen pitting attack on copper tube.
and Fig. 13 illustrates a carbon dioxide pitting attack on a copper tube.
Fig 13. Carbon dioxide pitting attack on copper tube
Stress Corrosion --This form of corrosion attacks the grain boundaries in stressed areas. Heat exchanger tubes usually have both avoidable and unavoidable residual stresses. These stresses are the result of drawing or forming the tube during manufacture, forming U bends, or expanding the tubes into tubesheets. Failures from this corrosion take the form of fine cracks, which follow lines of stress and material grain boundaries. Figure 14 shows stress corrosion failure in admiralty tubing
Fig. 14. Stress corrosion failure in admiralty tube
The corrodent that causes stress corrosion on stainless steel tubes is the chloride ion, which is potentially present in any compound formulated with chlorine. All naturally occurring waters contain the chloride ion in varying degrees. The chloride stress corrosion phenomenon is not well understood, but it is known that the frequency of occurrence rises with an increase in temperature and chloride ion concentration. Keeping tube wall temperatures below 115 F (calculated with maximum, not average. fluid temperatures) prevents stress corrosion cracking problems with chloride ion concentration up to 50 ppm. Figure 15 shows stress corrosion cracking in a type 304 stainless steel U bend.
Fig 15 Stress corrosion cracking in stainless steel U bend.
The corrodent that causes stress corrosion cracking on copper or copper alloy tubes is ammonia. Very small concentrations (1 ppm or less) can create a problem. The vacuum breaker used on steam-heated exchangers draws in ammonia from any leak in the ammonia refrigeration machine. The ammonia causes stress cracking problems, particularly in the inner U bends of the heat exchanger tubes. Copper-nickel alloys have good resistance to stress corrosion cracking and should be used in applications where low concentrations of ammonia are expected. Dezincification -- This problem occurs in copper-zinc alloys containing less than 85 percent copper when they are in contact with water having a high oxygen and carbon dioxide content, or in stagnant solutions. The effect tends to accelerate as temperature increases or pH decreases below 7. Dezincification creates a porous surface in which the zinc is chemically removed from the alloy. The remaining copper has a sponge-like appearance. Dezincification is prevented by using a brass with lower zinc content or a brass containing tin or arsenic to inhibit the chemical action, or by controlling the environment causing the problem.
Galvanic Corrosion -- This type of corrosion occurs when dissimilar metals are joined in the presence of an electrolyte, such as acidic water. Galvanic corrosion usually produces a higher rate of reaction on the less noble metal. For example, if a simple galvanic cell composed of copper and steel is immersed in a solution of sulfuric acid, the less noble steel corrodes quickly and the more noble copper is virtually unattacked
The galvanic chart shows the relative potential of materials to support this type of corrosion. Metals grouped together have relatively little tendency to produce galvanic corrosion: when two metals from substantially different groups are coupled together in an electrolyte, substantial corrosion of the less noble metal results. Figure 16 shows an example of galvanic corrosion on a steel baffle
Fig. 16. Galvanic corrosion on steal baffle
In this case, the less noble steel baffle was sacrificed at its point of contact with the copper tubing
Crevice Corrosion -- This type of corrosion originates in and around hidden and secluded areas, such as between baffles and tubes, Fig 17, or under loose scale or dirt.
Fig, 17. Crevice corrosion at contact between tube and baffle
A localized call develops and the resulting corrosion appears as a metal loss with local pits, often giving the impression that erosion is taking place. This condition is in contrast to a vibration failure in which the metal is sharply cut and there are no pits. Relatively stagnant conditions must exist for crevice corrosion to occur. The attack can often be controlled by making sure that velocities are adequate to prevent stagnation or the accumulation of solids. Condensate Grooving -- This problem occurs on the outside of steam-to-water heat exchanger tubes. particularly in the U-bend area. It is recognized by an irregular groove, or channel. cut in the tube as the condensate drains from the tubing in the form of rivulets. A corrosion cell usually develops in the wetted area because of the electrical potential difference between the dry and wet areas.
Figure 18 shows condensate grooving and the pitting attack.
Fig.18. Condensate grooving and pitting attack on tube
The condensate, which must be aggressive for this grooving to occur, wears away the protective oxide film as it drains from the tubing. Controlling condensate pH and dissolved gases and cleaning the tube bundle outside surface to remove oils that prevent uniform wetting of the tube usually reduce the problem potential. Combination Mechanical Failure and Chemically Induced Corrosion -- In many instances heat exchanger failure is not from a single cause, but a combination of more than one condition. For example, a pitting attack, galvanic attack, and crevice corrosion attack could occur simultaneously at one or more locations. Quite often, a mechanical problem combining with a corrosion problem produces a quicker failure than either of them alone. There are two common types of combination mechanical and corrosion failures; erosion-corrosion and corrosion-fatigue.
Erosion-Corrosion -- Any corrosion is greatly accelerated if the protective films are worn away by excessive velocity, suspended solids, or mechanical vibration. Erosion-corrosion is usually found in the entrance area of tubes, below the shell inlet nozzle, at the point of contact with baffles and tubes, and inside the U-bend area of tubes, particularly the tighter bends, Figure 19 shows an erosion-corrosion attack. The bright copper surface at the tube entrance is erosion; the yellowish deposit inside the tube is corrosion.
Fig. 19. Erosion-corrosion attack on tube
Corrosion-Fatigue -- In this dual failure mode, stresses associated with fatigue are the result of externally applied mechanical loads- such as vibrations from machinery, expansion or contraction because of temperature cycles, or light water hammer. In most environments where only corrosion occurs, corrosion products and films block or retard further attack.
However, in corrosion-fatigue, Fig. 20, cyclic stresses rupture the protected areas and make them permeable; this action subjects open areas to accelerated corrosion. Improperly supported tube bundles in domestic hot water storage tanks often suffer from this kind of failure.
Fig. 20. Corrosion-fatigue attack on tube.
Vibrations may not be very severe: however, resulting stresses act additively to the existing internal tensile stresses. The stress level is raised beyond the strength of the material and cracking or a fracture results.
Scale, Mud, and Algae Funding- Various marine growths deposit a film or coating on the surfaces of heat transfer tubes. The film acts as an insulator, restricting heat flow and protecting corrodents. As a result of this insulating effect, tube wall temperatures go up and corrosion increases. Scale is the result of dissolved minerals precipitating out of heat transfer fluids. The solubility of these minerals is altered by forces within the heat exchanger, such as changes in temperature or chemical reactions. For example, when calcium bicarbonate, a common constituent of many waters, is heated, carbon dioxide is released and the material is reduced to calcium carbonate, a relatively insoluble compound that precipitates and coats heat transfer surfaces. Experience shows that the rate of precipitation is reduced with increasing find velocity. Fluid velocity must be matched to the tube material's ability to withstand the erosive effects of velocity. Suspended solids are usually found in the form of sand. iron, silt, or other visible particles in one or both of the heat transfer fluids, If velocities are not high enough to keep them in suspension, particles settle out, causing the same kinds of problems associated with scale from dissolved solids. In addition, suspended solids are very abrasive to tubing and other heat exchanger parts. If abrasive suspended solids are handled in the heat exchanger, fluid velocity must be kept low enough to prevent erosion. Algae and other marine growths are a serious problem if they get in the heat exchanger. In many cases, the environment in the heat exchanger is conducive to rapid proliferation of the algae or other marine growths. which restrict flow and impede heat transfer. Chemical algicides, such as chlorine. are effective in controlling algae and other marine growths; high fluid velocities also discourage their attachment and expansion.
http://www.deppmann.com/home/wp-content/uploads/2016/10/4-Types-of-Heat-Exchanger-Failures-article.pdf













Good post! We are linking to this particularly great post on our website.
RispondiEliminaStainless Steel Corner Trim
Corner Profile
Stainless Steel Decorative Trim
Good Information about Heat Exchangers. To know more click on
RispondiElimina90 10 Cupro Nickel Tubes